New study shows longitudinal biomarker trajectories support personalized patient management
Manassas, Virginia--(Newsfile Corp. - August 26, 2025) - A new study published in Ophthalmology Science validates that artificial intelligence (AI) can accurately track disease activity in patients with wet age-related macular degeneration (AMD) by analyzing up-to daily home OCT images.1 This approach adds a new dimension to patient management by allowing physicians to follow biomarker trajectories over time and assess response to treatment more effectively.
The SCANLY Home OCT enables patients to perform daily retinal imaging from home. Using these frequent images, the AI-based Notal OCT Analyzer (NOA) estimates disease biomarkers. These measurements are then plotted over time, giving physicians a clearer picture of how the disease is progressing or responding to therapy.
The study evaluated data from 180 wet AMD patients and compared the NOA-generated outputs to annotations made by three expert graders. The results showed high agreement between NOA and human experts in identifying clinically meaningful changes. The study also found that using personalized thresholds to identify changes in a patient's retinal anatomy resulted in higher sensitivity than applying a single, fixed threshold across all patients. When physicians set individualized thresholds, sensitivity increased from about 90% to 99.1% without increasing false positives.
"Home OCT-based monitoring has been validated in large pivotal trials with over 500 patients, which is rare for medical imaging technology," said Dr. Theodore Leng, Director of Clinical and Translational Research and Ophthalmic Diagnostics at Stanford's Byers Eye Institute. "Our study went a step further by showing that home OCT longitudinal data trajectories are clinically valuable and essential in helping retina specialists personalize disease management."
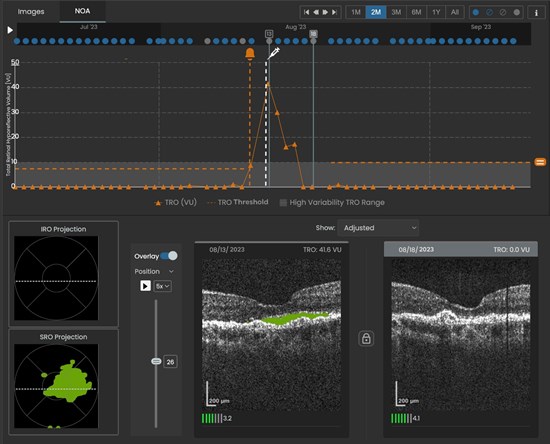
Fig. 1 SCANLY Home OCT longitudinal disease biomarker volume trajectory with notification and treatment indicators (top) and corresponding projection map of subretinal hypo-reflective (SRO) spaces and B-scans (bottom).
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/10516/263497_5733f56664fca921_001full.jpg
Previous pivotal trials for SCANLY Home OCT demonstrated that home-acquired images are equivalent to in-office OCT for visualizing key biomarkers in wet AMD patients.2 Those trials also validated NOA for estimating hypo-reflective spaces, a key disease biomarker, in a cross-sectional setting.3 This latest study is the first to examine NOA's performance in tracking changes over time in a home setting.
"This work is a major step forward in personalized AMD patient care," said Kester Nahen, PhD, CEO of Notal Vision. "It shows that home imaging, powered by AI and guided by physicians, can reliably track disease activity and help doctors make informed patient management decisions."
The study results will be presented by Dr. Leng at the Retina Society meeting in Chicago on September 12, 2025.
References:
- Leng, Theodore, et al. "Longitudinal Validation of the Artificial Intelligence Algorithm in Home OCT for Age-Related Macular Degeneration. Report 3." Ophthalmology Science (2025): 100907.
- Heier, Jeffrey S., et al. "Pivotal Trial Validating Usability and Visualization Performance of Home OCT in Neovascular Age-related Macular Degeneration. Report 1." Ophthalmology Science, Volume 5, Issue 5, (2025): 100772. https://doi.org/10.1016/j.xops.2025.100772.
- Schneider, Eric W., et al. "Pivotal Trial Toward Effectiveness of Self-administered OCT in Neovascular Age-related Macular Degeneration. Report 2-Artificial Intelligence Analytics." Ophthalmology Science 5.2 (2025): 100662.
###
About Notal Vision
Notal Vision is a patient-centric ophthalmic remote monitoring services provider extending care from the clinic to the home. We empower physicians with innovative home-based technologies and remote monitoring services that support patient management between office visits. Our solutions combine self-operated digital diagnostic devices, AI-enabled data analysis, and a physician-led monitoring center-all with the goal of helping preserve patients' vision. Learn more at www.notalvision.com.
The Medicare-accredited and ophthalmologist-led Notal Vision Monitoring Center is dedicated to remote monitoring and patient engagement. Staffed by certified ophthalmic professionals, the center provides nationwide service for age-related macular degeneration (AMD) monitoring.
Notal Vision offers two distinct remote monitoring solutions:
- ForeseeHome AMD Monitoring Program: Designed for patients with intermediate dry AMD, this FDA-cleared and Medicare covered program uses an at-home, AI-powered visual function test to detect changes that may indicate conversion from dry to wet AMD. The Monitoring Center alerts physicians to potential disease progression-often before the patient notices symptoms-enabling earlier intervention and improved outcomes.
- SCANLY Home OCT Monitoring Service: Indicated for patients with diagnosed wet AMD, the first-in-class, FDA-cleared SCANLY allows technician-free OCT imaging from home. Patients perform regular self-scans using an intuitive device with AI-assisted image analysis. The physician receives 24/7 access to annotated OCT B-scans and automated alerts when clinically relevant changes are detected, enabling timely office visits.
Together, these programs support physicians across the full spectrum of AMD care-from early detection to ongoing disease management.
Media Contact:
Candice Morin
candicem@notalvision.com

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/263497