PALO ALTO, CA / ACCESS Newswire / May 30, 2025 / Riboscience, LLC, today announced the presentation of data from the completed dose escalation Phase 1a of the Phase 1a/b clinical trial of the ENPP1 inhibitor RBS2418 that will be delivered by Dr. Thomas U. Marron, MD, PhD, from Mount Sinai, New York at the American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago on June 2, 2025. RBS2418 is the first ENPP1 inhibitor in clinical development.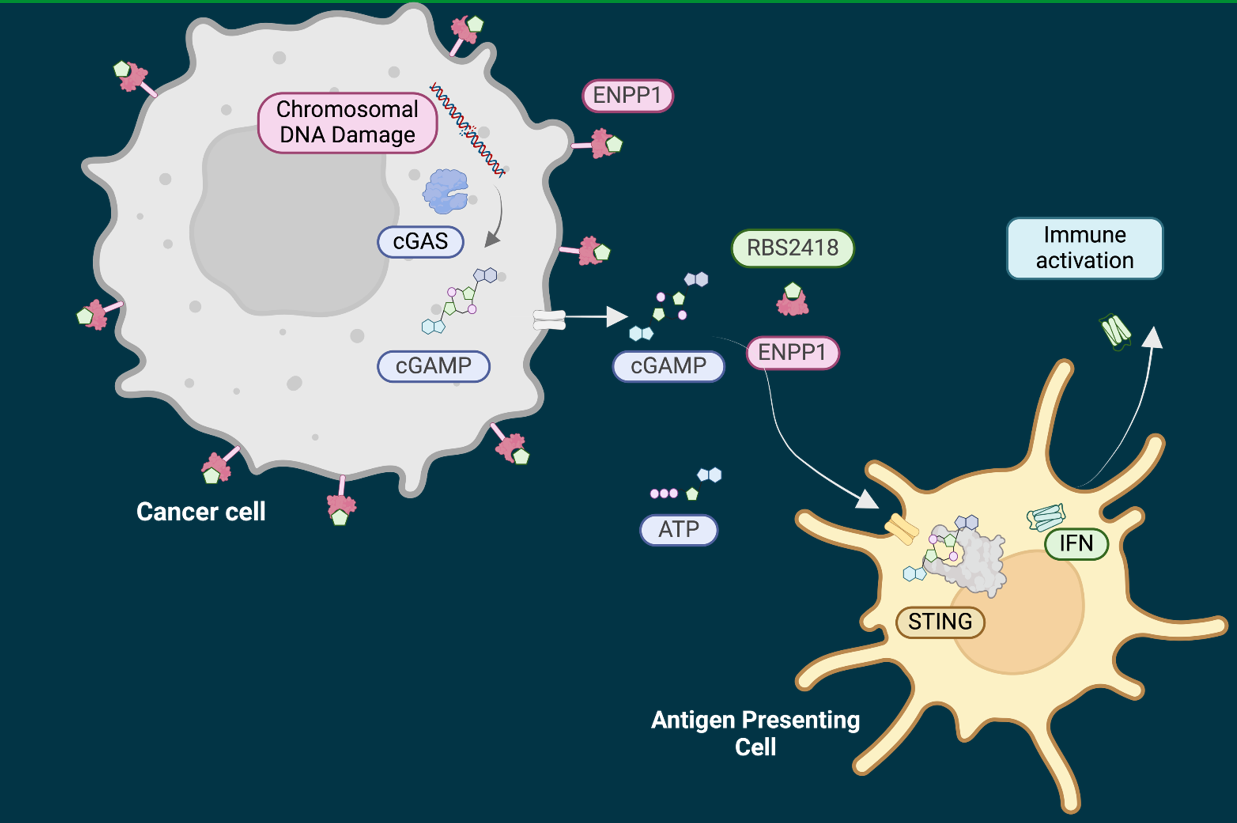
RBS2418 inhibits ENPP1 catalyzed ATP and cGAMP hydrolysis and enables cGAMP-mediated immune activation
Clinical study RBS2418-1001 (NCT05270213) is a Phase 1, open-label, non-randomized study in adult patients with advanced, unresectable, recurrent or metastatic solid tumors, who have progressed on, or are ineligible for standard therapies. The dose escalation phase of the study followed a 3+3 design with increasing doses of RBS2418 given orally as monotherapy or in combination with Pembrolizumab.
At ASCO, Riboscience is presenting data from the completed Phase 1a trial of its new broad-spectrum immunotherapy for patients with advanced cancers, highlighting its safety and immune activation potential.
The ASCO presentation #2577 summarizes the safety, PK/PD results and clinical outcomes of treatment with RBS2418 from the 24 patients in the dose escalation phase of the study. There are 13 different cancer types represented and 83% of patients had failed 3 or more lines of prior treatment. The concentration of RBS2418 in plasma and in tumor samples exceeded the human serum EC90 of ENPP1 inhibition in all patients at all dose levels (100, 200, 400, 800 mg). Median plasma C max and C trough levels of RBS2418 increased dose-proportionally. No DLTs, treatment-related SAEs or treatment-related AEs above grade 2 have been observed to date. The subject with the longest time on treatment is continuing daily 800 mg BID RBS2418 monotherapy treatment for >17 months at this time, confirming long-term safety and immune control. Baseline cold tumor phenotype and immune activation and tumor infiltration of CD4 and CD8 cells on treatment correlated with the expression of ENPP1 and cGAS protein in baseline tumor samples (EG+ phenotype).
Progression-free survival (PFS) was significantly increased in EG+ vs EG- phenotypes (p=0.001). Clinical benefit from RBS2418 was dependent on the presence of the drug target (ENPP1), indicating target-inhibition dependent clinical benefit with RBS2418 treatment, consistent with the PK/PD results that demonstrated full ENPP1 inhibition and cGAMP stabilization at all dose levels.
These results show a target-inhibition-dependent immune activation and significant clinical benefit of a well-tolerated oral RBS2418 treatment and support further clinical development of this novel first-in-class immunotherapy agent. The first Phase 2 study of RBS2418 treatment of advanced metastatic Colorectal Cancer has started enrolling participants in February 2025 (NCT06824064).
ABOUT RIBOSCIENCE
Riboscience is a clinical-stage biotechnology company developing treatments for serious infectious diseases and cancer. We apply ribose and structure-guided design technologies to the discovery of drugs that target proteins that are essential and unique to specific infectious pathogens or anti-tumor immune response. Riboscience is headquartered in the San Francisco Bay Area with laboratories in Sunnyvale and Palo Alto.
Contact Information
Klaus Klumpp
President and Founder,
Riboscience
email: Klaus.klumpp@riboscience.com
Ph no. 6502834398
SOURCE: Riboscience
Related Images
View the original press release on ACCESS Newswire




