Stocks trading under $10 rarely come with the potential for life-changing gains, especially in today’s cautious market. In most sectors, that kind of upside is almost unheard of. Some biotech stocks, however, are an exception. When encouraging clinical data and regulatory milestones align, a single breakthrough can rapidly reprice a biotech stock. That is precisely why Wall Street expects this under-$10 biotech company to skyrocket by as much as 404% in 2026. Let’s find out why.
About Arcturus Therapeutics
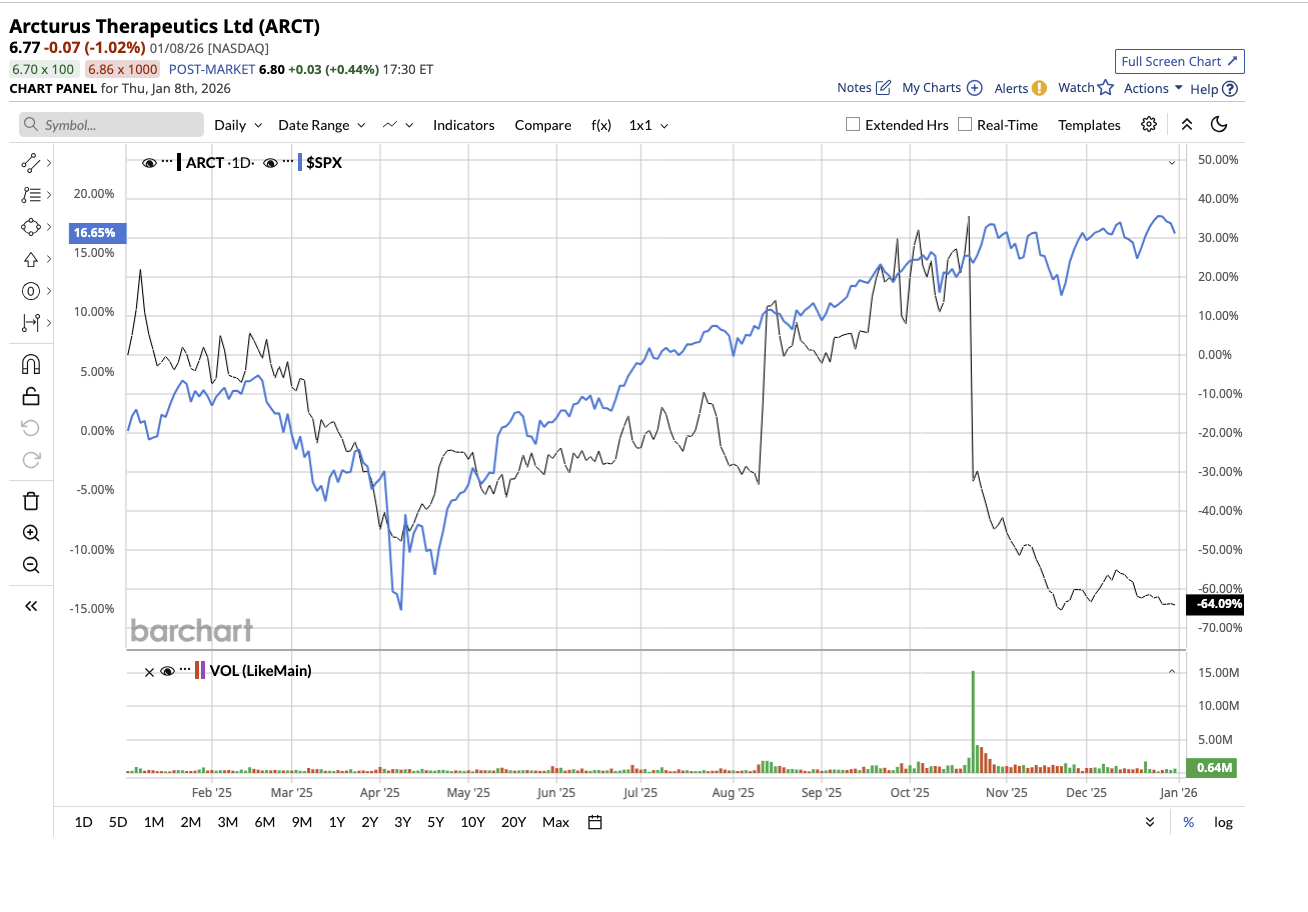
Arcturus Therapeutics (ARCT) is a biotechnology company that develops messenger RNA (mRNA)-based medicines and vaccines. Instead of treating symptoms, Arcturus seeks to address the root cause of the problem by helping the body make the right proteins on its own. Valued at $194.3 million, ARCT stock dipped 64% last year, compared to the S&P 500 Index ($SPX) gain of 16%. However, the stock is up 10% so far this year.
The company has already developed the world’s first approved self-amplifying mRNA Covid-19 vaccine, KOSTAIVE. It continues to collaborate with global partners to develop mRNA-based vaccines for Covid-19 variants and pandemic influenza. It is also working on developing mRNA therapies for cystic fibrosis (CF) and ornithine transcarbamylase (OTC) deficiency, a serious inherited disorder.
A High-Risk, High-Reward mRNA Story Taking Shape
Arcturus is emerging as one of the most closely watched under-$10 biotech stocks heading into 2026, driven by encouraging clinical data, a clearer pipeline roadmap, and aggressive cost controls that extend its financial runway. Momentum is building around ARCT-032, Arcturus’ inhaled mRNA therapeutic candidate for cystic fibrosis. Interim Phase 2 data released in October indicated that daily 10 mg treatment for 28 days in six Class I CF adults was generally safe and well tolerated. Importantly, protocol-specified high-resolution CT scan analysis employing FDA-cleared AI technology revealed reductions in mucus burden in four of the six patients.
Building on these early indications, Arcturus intends to launch a 12-week safety and preliminary efficacy study in up to 20 CF patients in the first half of 2026. In addition, the company is enrolling a third cohort to study a higher 15 mg daily dose over 28 days, which will help refine the program's dose-response profile. These next trial steps are essential since positive findings will considerably bolster the ARCT-032 investment thesis.
Beyond CF, Arcturus is developing ARCT-810 for OTC deficiency, a rare genetic metabolic condition. The company aims to collaborate with regulatory agencies on pivotal trial designs for both pediatric and adult populations in the first half of 2026, ensuring that this second key product remains on schedule. Arcturus’ platform continues to be validated through its vaccine programs. In Japan, partner Meiji Seika Pharma issued an enhanced two-dose vial of KOSTAIVE targeting the JN.1 variant XEC in August 2025, following regulatory approval. Additional Phase 3 and Phase 1 trials on Covid-19 and pandemic influenza candidates indicated significant immune responses, excellent safety profiles, and support for continued development of the company's STARR self-amplifying mRNA technology. These data highlight the versatility of Arcturus’ mRNA platform across both therapeutics and vaccines.
Arcturus' main source of revenue for now is through licensing, consulting, other technology transfer fees, and collaboration fees from R&D partnerships with other biotech companies. In the third quarter, revenue came in at $17.2 million, with net loss standing at $13.5 million. At the end of the third quarter, Arcturus reported $237.3 million in cash, cash equivalents, and restricted cash. With additional cost cuts planned for the fourth quarter and the Phase 3 CF trial delayed until 2027, management anticipates the company's financial runway to extend into 2028. This gives the company plenty of time to pursue its major programs without having to worry about finance in the foreseeable future.
While biotech investing always carries significant risk, Arcturus' favorable interim CF data, a larger Phase 2 study starting in 2026, regulatory alignment for a rare illness program, and a stronger balance sheet make a compelling argument for a high-risk, high-reward candidate.
What Does Wall Street Say About ARCT Stock?
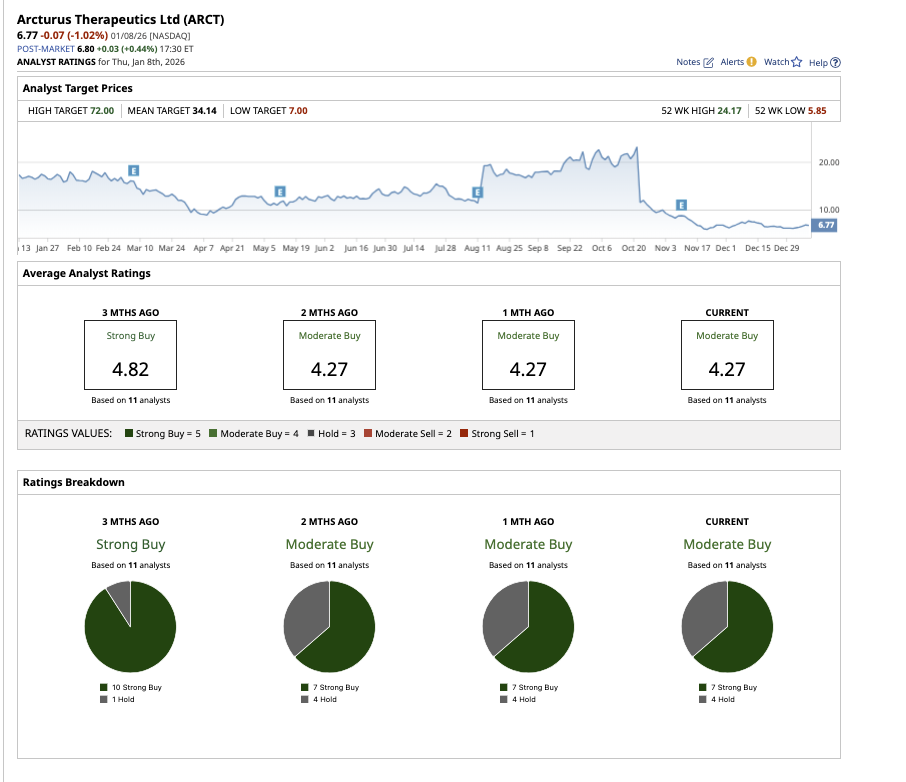
Overall, Wall Street rates ARCT stock a “Moderate Buy.” Out of the 11 analysts covering the stock, seven rate it a “Strong Buy” and four rate it a “Hold.” The average target price of $34.14 suggests the stock can rally as much as 404% over current levels. Plus, the high target price of $72 proposes upside potential of 963% over the next 12 months. While the upside may seem far-fetched, biotech stocks are known for rallying sky-high once a successful product hits the market.
On the date of publication, Sushree Mohanty did not have (either directly or indirectly) positions in any of the securities mentioned in this article. All information and data in this article is solely for informational purposes. For more information please view the Barchart Disclosure Policy here.
More news from Barchart
- 1 Under-$10 Stock Set to Surge as Much as 963% in 2026
- The Saturday Spread: Reading the Market’s Signals to Jump Ahead of the Crowd (HPE, SNOW, CRWD)
- Wall Street’s Top Warren Buffett Dividend Stocks to Buy Now
- Nvidia CEO Jensen Huang Warns Investors That the AI Market Is Bigger Than They Realize With Over ‘One and a Half Million AI Models in the World’





