PYX-201 Phase 1 Part 1 trial progressing with initial data expected in the fall of 2024
PYX-106 Phase 1 trial progressing with initial data expected 2H 2024
Completed $50 million private placement
Expected cash runway into 2H 2026
BOSTON, March 21, 2024 (GLOBE NEWSWIRE) -- Pyxis Oncology, Inc. (Nasdaq: PYXS), a clinical stage company focused on developing next generation therapeutics to target difficult-to-treat cancers, today reported financial results for the year and quarter ended December 31, 2023, and provided a corporate update.
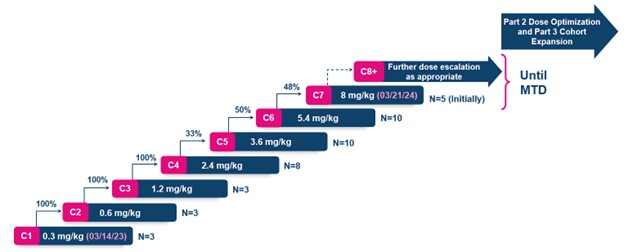
PYX-201, a first-in-concept tumor stroma targeting antibody-drug conjugate (ADC) against the stromal Extradomain-B Fibronectin (EDB+FN) target, has dosed 37 patients in 6 cohorts since initiating the Phase 1 trial in March 2023. PYX-201 recently cleared the 21-day Dose Limiting Toxicity (DLT) observation period for ten subjects in Cohort 6 at a dose of 5.4 mg/kg. The Dose Escalation Steering Committee (DESC) met on March 19, 2024, and voted to escalate dosing into Cohort 7 at a dose of 8 mg/kg.
PYX-201 has been well tolerated to date, with no significant evidence of target mediated toxicities experienced by the 37 subjects enrolled and dosed. Approximately 54% of subjects have experienced grade 2, and 6% of subjects have experienced grade 3 treatment emergent adverse events (TEAEs). No subjects have reported TEAEs leading to dosing delay or study drug discontinuation. Another 10-15 subjects are likely to be dosed at either Cohort 7 (8 mg/kg) or future higher dose level cohorts, should PYX-201's profile continue to support further dose escalation.
As we continue to dose escalate, we are focusing ongoing enrollment on four tumor types of high interest identified through the assessment of several factors, including, but not limited to, IHC target expression data, stromal volume, and unmet medical need: head and neck squamous cell carcinoma (HNSCC), non-small cell lung cancer (NSCLC), ovarian cancer, and pancreatic ductal adenocarcinoma cancer (PDAC).
Dose escalation and subject numbers by dose since initiating the trial in March 2023
“We believe the encouraging PYX-201 safety profile observed to date likely reflects the specificity of target expression within tumor tissue and the potential for a wider therapeutic index given the novel mechanism of action within the tumor microenvironment,” said Lara S. Sullivan, M.D., President and Chief Executive Officer of Pyxis Oncology. “The tumor stroma is a prominent component of many solid tumors, and we believe that PYX-201 could have broad utility in many cancer settings. The global study remains on track with continued investigator enthusiasm and ease of enrollment, and we look forward to announcing initial results in the fall of 2024 with final timing dependent on ongoing continued dose escalation and finalization of subject scans.”
Dr. Sullivan added, “We are also continuing to enroll our Phase 1 study evaluating PYX-106, a fully human immunotherapy antibody candidate that is designed to block the activity of Siglec-15 in subjects with NSCLC and other tumors of interest. We look forward to initial results from this program in 2H 2024.”
Program and Corporate Updates
- PYX-201 in the PYX-201-101 trial: To date, 37 subjects have been dosed, and we are currently enrolling Cohort 7 at 8 mg/kg. In the fall of 2024, we plan to report efficacy, safety, pharmacokinetics (PK), pre-clinical insights, the plan for the next development phase, and the likely timing of associated catalysts.
- PYX-106 in the PYX-106-101 trial: Phase 1 trial focusing on NSCLC and other tumor types. Study dosing is ongoing with 21 subjects dosed to date and Cohort 5 is fully enrolled at 8 mg/kg administered once every two weeks. Preliminary data is anticipated in 2H 2024.
- In Feb. 2024, completed a $50M PIPE with participation from new and existing institutional investors, including Deep Track Capital, Ridgeback Capital Investments L.P., Blue Owl Healthcare Opportunities, Laurion Capital Management, and StemPoint Capital L.P. Pyxis Oncology intends to use the proceeds to fund the continued development of PYX-201 and for working capital and general corporate purposes.
Anticipated Upcoming Milestones
- PYX-201: Report preliminary Phase 1 data and PK/PD results in fall of 2024
- PYX-106: Report preliminary Phase 1 data and PK/PD results in 2H 2024
Full Year and Q4 2023 Financial Results
- As of December 31, 2023, Pyxis Oncology had cash and cash equivalents, including restricted cash, and short-term investments of $120.8 million. Following the end of fiscal year 2023, we raised gross proceeds of $10.8 million via ATM offering and completed $50 million of private placement. Pyxis Oncology expects to have the resources to fund operations into 2nd half of 2026.
- Research and development expenses were $49.6 million for the year ended December 31, 2023, compared to $86.1 million for the year ended December 31, 2022. The decrease was primarily due to a one-time payment of $17.3 million to acquire exclusive licensing rights for the FACT platform, one-time payment of $10 million to acquire licensing rights to PYX-106 and decrease in contract manufacturing costs for drug products and drug substances by $12.5 million in 2022. This decrease was partially offset by a $5.0 million increase in clinical trial related expenses for our ongoing Phase 1 clinical trials for PYX-201 and PYX-106.
- General and administrative expenses were $32.6 million for the year ended December 31, 2023, compared to $37.4 million for the year ended December 31, 2022. The decrease was primarily related to a reduction in professional and consultant fees partially offset by higher personnel-related expenses, including stock-based compensation.
- Net loss was $73.8 million, or ($1.85) per common share, for the year ended December 31, 2023, compared to $120.7 million, or ($3.65) per common share, for the year ended December 31, 2022. Net losses for the years ended December 31, 2023 and 2022, included $16.9 million and $15.8 million, respectively, related to non-cash stock-based compensation expense.
- As of March 20, 2024, the outstanding number of shares of Common Stock of Pyxis Oncology was 58,133,375.
About Pyxis Oncology, Inc.
Pyxis Oncology, Inc. is a clinical stage company focused on defeating difficult-to-treat cancers. The company is efficiently building next generation therapeutics that hold the potential for mono and combination therapies. PYX-201, an antibody-drug conjugate (ADC) that uniquely targets EDB+FN within the tumor stroma, and PYX-106, a fully human Siglec-15-targeting antibody designed to block suppression of T-cell proliferation and function, are being evaluated in ongoing Phase 1 clinical studies in multiple types of solid tumors. Pyxis Oncology’s therapeutic candidates are designed to directly kill tumor cells and to address the underlying pathologies created by cancer that enable its uncontrollable proliferation and immune evasion. Pyxis Oncology’s ADC and immuno-oncology (IO) programs employ novel and emerging strategies to target a broad range of solid tumors resistant to current standards of care. To learn more, visit www.pyxisoncology.com or follow us on Twitter and LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. These statements are often identified by the use of words such as “anticipate,” “believe,” “can,” “continue,” “could,” “estimate,” “expect,” “intend,” “likely,” “may,” “might,” “objective,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “to be,” “will,” “would,” or the negative or plural of these words, or similar expressions or variations, although not all forward-looking statements contain these words. We cannot assure you that the events and circumstances reflected in the forward-looking statements will be achieved or occur and actual results could differ materially from those expressed or implied by these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those identified herein, and those discussed in the section titled “Risk Factors” set forth in Part II, Item 1A. of the Company’s Annual Report on Form 10-K filed with SEC on March 21, 2024, and our other filings, each of which is on file with the Securities and Exchange Commission. These risks are not exhaustive. New risk factors emerge from time to time, and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date hereof and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and investors are cautioned not to unduly rely upon these statements. Except as required by law, we undertake no obligation to update any forward-looking statements to reflect events or circumstances after the date of such statements.
Pyxis Oncology Contact
Pamela Connealy
CFO and COO
ir@pyxisoncology.com
---tables to follow---
PYXIS ONCOLOGY, INC.
Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share amounts)
| Year Ended December 31, | |||||||
| 2023 | 2022 | ||||||
| Operating expenses: | |||||||
| Research and development | $ | 49,586 | $ | 86,129 | |||
| General and administrative | 32,610 | 37,352 | |||||
| Total operating expenses | 82,196 | 123,481 | |||||
| Loss from operations | (82,196 | ) | (123,481 | ) | |||
| Other income, net: | |||||||
| Interest and investment income | 6,630 | 2,764 | |||||
| Sublease income | 1,776 | — | |||||
| Total other income, net | 8,406 | 2,764 | |||||
| Net loss | $ | (73,790 | ) | $ | (120,717 | ) | |
| Net loss per common share - basic and diluted | $ | (1.85 | ) | $ | (3.65 | ) | |
| Weighted average shares of common stock outstanding - basic and diluted | 39,904,603 | 33,033,081 | |||||
PYXIS ONCOLOGY, INC.
Consolidated Balance Sheets
(In thousands)
| December 31, | |||||||
| 2023 | 2022 | ||||||
| Assets | |||||||
| Current assets: | |||||||
| Cash and cash equivalents | $ | 9,664 | $ | 179,293 | |||
| Marketable debt securities, short-term | 109,634 | — | |||||
| Restricted cash | 1,472 | 1,472 | |||||
| Prepaid expenses and other current assets | 3,834 | 5,847 | |||||
| Total current assets | 124,604 | 186,612 | |||||
| Property and equipment, net | 11,872 | 11,165 | |||||
| Intangible assets, net | 24,308 | — | |||||
| Operating lease right-of-use assets | 12,942 | 13,602 | |||||
| Total assets | $ | 173,726 | $ | 211,379 | |||
| Liabilities and Stockholders’ Equity | |||||||
| Current liabilities: | |||||||
| Accounts payable | $ | 3,896 | $ | 7,097 | |||
| Accrued expenses and other current liabilities | 12,971 | 24,537 | |||||
| Operating lease liabilities, current portion | 1,232 | — | |||||
| Deferred revenue | 7,660 | — | |||||
| Total current liabilities | 25,759 | 31,634 | |||||
| Operating lease liabilities, net of current portion | 20,099 | 18,921 | |||||
| Deferred tax liability, net | 2,164 | — | |||||
| Total liabilities | 48,022 | 50,555 | |||||
| Commitments and contingencies | |||||||
| Stockholders’ equity: | |||||||
| Preferred stock | — | — | |||||
| Common stock | 45 | 34 | |||||
| Additional paid-in capital | 411,821 | 373,225 | |||||
| Accumulated other comprehensive income | 63 | — | |||||
| Accumulated deficit | (286,225 | ) | (212,435 | ) | |||
| Total stockholders’ equity | 125,704 | 160,824 | |||||
| Total liabilities and stockholders’ equity | $ | 173,726 | $ | 211,379 | |||







