BERN, CH / ACCESS Newswire / April 24, 2025 / LEM Surgical, a developer of advanced robotic technologies for hard tissue surgery, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its Dynamis Robotic Surgical System, marking a significant milestone in the evolution of robotic-assisted hard tissue surgery.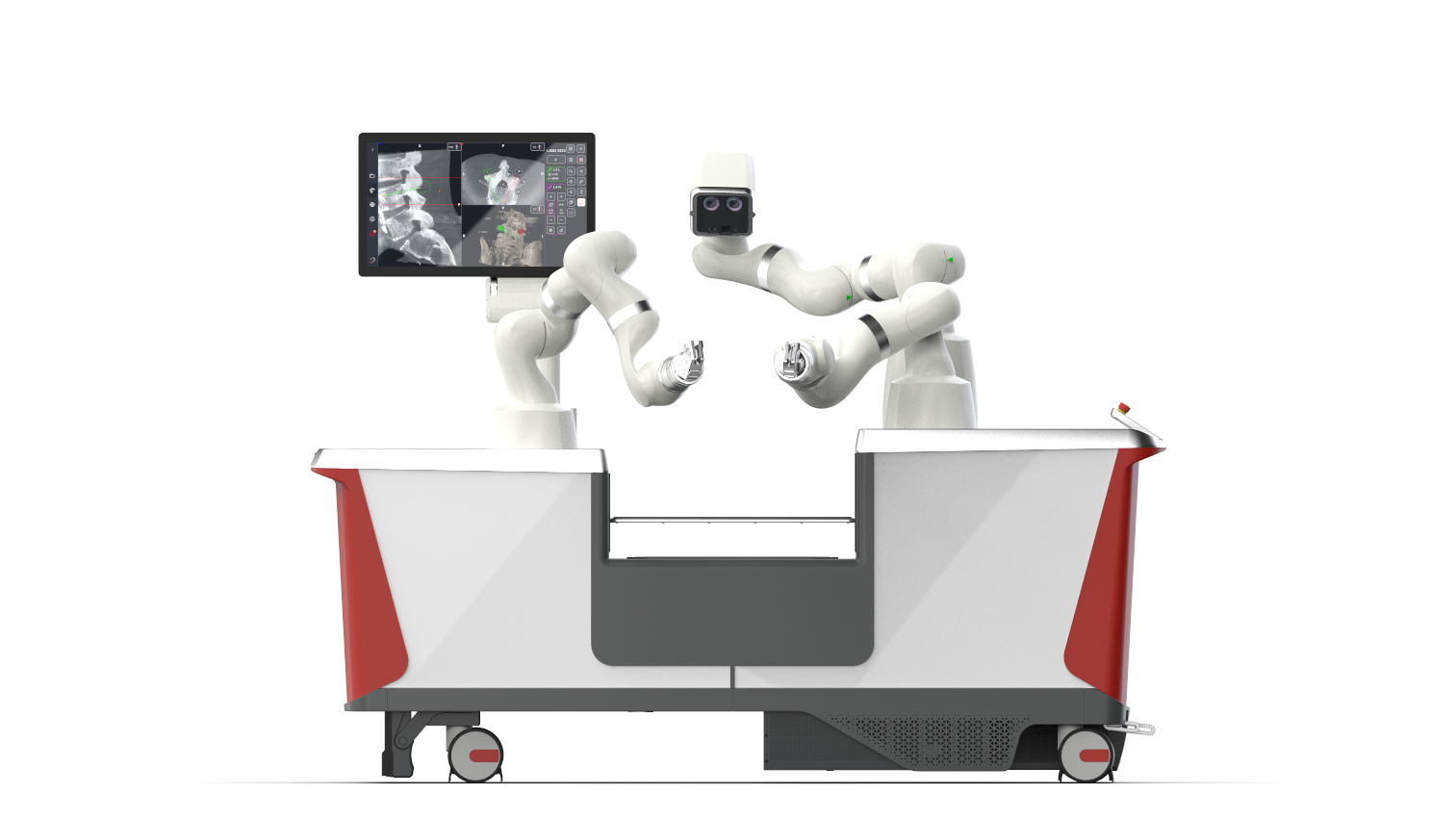
Dynamis Robotic Surgical System for Hard Tissue Surgery
The Dynamis Robotic Surgical System is an integrated navigation-based robotic platform designed to enhance the accuracy and control during spine surgeries. With this clearance, U.S. spine surgeons will now have access to a next-generation technology that combines real-time imaging, dynamic guidance, and adaptable instrumentation compatibility within a single, versatile platform.
"This FDA clearance of the Dynamis Robotic Surgical System is a testament to the hard work of our team and our commitment to launching robotic surgery platforms that benefit the healthcare system and improve clinical outcomes," said Yossi Bar, CEO of LEM Surgical. "We are very proud to introduce to the world the first-of-its-kind, multi-arm surgical robot for hard tissue surgery. The next generation of intelligent and highly capable robots for the operating room has begun, and LEM Surgical is positioned to lead the way."
The Dynamis Robotic Surgical System features three robotic arms - two for surgical guidance and one for optical navigation - consolidated into a single cart that fits partially beneath the surgical table. Unlike currently available systems, the Dynamis Robotic Surgical System supports a wide range of surgical instruments through its unique adjustable end-effectors and intraoperative qualification process, enhancing compatibility and hospital workflow efficiency.
"Our vision is to advance the field of robotic surgery," Chris Prentice, Chief Commercial Officer of LEM Surgical, added. "The Dynamis Robotic Surgical System will be introduced in America at select hospitals this year, and we will increase commercialization of the system in 2026."
LEM Surgical is headquartered in Bern, Switzerland, and is dedicated to advancing robotic solutions that elevate standards of care across hard tissue procedures. LEM Surgical's U.S. offices and the Dynamis Robotic Surgical System Demonstration Suite are in Tampa, Florida.
For more information, visit www.lemsurgical.com or contact communications@lemsurgical.com.
Contact Information
Yossi Bar
Chief Executive Officer
communications@lemsurgical.com
SOURCE: LEM Surgical AG
View the original press release on ACCESS Newswire





