SAN DIEGO, CA / ACCESSWIRE / December 7, 2021 / Ainos, Inc. ("we", "our", or the "company"), (OTC PINK:AIMD), a diversified healthcare company engaged in the discovery and development of pharmaceutical, medical devices and biotech products announced it entered into a Development and Sales Agreement with InnoPharmax, Inc. ("InnoPharmax"), a specialty pharmaceutical company focused in the development and commercialization of products for the treatment of infectious diseases, immunology, and oncology.
Ainos and InnoPharmax agreed to jointly develop and promote an orally administered cytotoxin-induced complementary combined therapy ("CICCT") for the treatment of COVID-19 and potentially other viral infections. The companies plan to develop this combined therapy leveraging Ainos' VELDONA® drug platform based on low-dose oral interferon along with InnoPharmax's orally administered antiviral drug, GemOral®.
"We believe that CICCT combined with interferon may increase the production of human cytotoxin, an autoimmune hormone that is reported to inhibit viruses," said Ainos' CEO Chun-Hsien Tsai. "The high level of COVID-19 cases -- still nearly 100,000 new confirmed cases despite increasing vaccination rates reported each day and more than 1,000 confirmed deaths during the past week in the U.S. alone according to the U.S. Department of Health and Human Services - suggests that the world needs a broader range of effective antiviral therapies," he observed.
"For COVID-19 patients we are particularly focused on cytokine storm, an imbalanced innate immunity response that causes severe inflammation in the lungs and necrosis associated with a high IL-6/IFN ratio," remarked Chun-Hsien Tsai. "We believe a combined therapy of Ainos' VELDONA® drug therapeutic and InnoPharmax's D07001 GemOral® can effectively increase the secretion of interferon in the human body by stimulating secretion in vivo and in vitro, thereby inhibiting the replication of coronavirus and effectively enhancing immunoreaction," Mr. Tsai said.
The companies plan to jointly perform animal and investigator-initiated trials for safety and efficacy testing in Taiwan early next year and conduct a subsequent Phase III clinical trial with the goal of applying for regulatory approvals, including U.S. emergency use authorization if available, sometime in the third quarter of 2022. Upon commercialization, Ainos will be responsible for global sale in countries for which the product is approved for uses, and InnoPharmax will be responsible for drug production and manufacturing. The two parties will share profits on drug sales.
"Given the current market for other similar proposed antiviral regimens, we assume the cost per treatment globally to be approximately USD 500, subject to change in accordance with market dynamics," Mr. Tsai said.
According to a press release by the U.S. National Institutes of Health published on October 18, 2021, "Laboratory studies have shown that the normal type 1 interferon response is suppressed after infection with SARS-CoV-2, the virus that causes COVID-19. In addition, previous studies of hospitalized patients with COVID-19 demonstrated reduced production of interferon in response to SARS-CoV-2 infection in many patients, and this was associated with more severe disease."
Ainos' VELDONA® is a low-dose oral interferon alpha (IFN-α) therapeutic, with the ability to "interfere" with viral replication by protecting cells from virus infections, spread and cancer cell division. Interferon alpha (IFN-α) also has various antitumor activities including the direct induction of cytotoxicity and the activation of NK cells and antibody-dependent cell-mediated cytotoxicity.
Studies have shown that type I interferon, IFN-α, (B lymphocytes and monocyte) and IFN-β (fibroblast) not only directly inhibits the replication of virus cells but also stimulates the operation of the immune system. Type II interferons, including IFN-γ (T lymphocyte), have the functions of antivirus, activating phagocytes and inducing expression of MHC molecular. We plan to investigate combining the functions of type I and type II interferons with the hopes of more effectively inhibiting the replication of virus and enhance immunoreaction. We are focused on reducing the continuous secretion of inflammatory cytokine to avoid severe pneumonia reactions and cytokine storms.
InnoPharmax's oral drug D07001 GemOral® inhibits inflammatory cytokine, such as IL-6 and CCL2, and also induces the production of interferon-stimulated gene (ISG) and interferon (IFN-b and IFN-λ). Gemcitabine, a key compound in GemOral®, can improve the performance of type II interferon-γ and CD8+ of immune cells. Gemcitabine can replace cytidine to be embedded in DNA or RNA during the process of nucleotide replication, resulting in failure of nucleic acid replication and cytotoxicity which is beneficial to inhibit the replication and the growth of virus in the body.
Overview of VELDONA®
- VELDONA® may be superior to high dose injectable interferon in safety, cost and ease of administration.
- Administered sublingually as small tablet (lozenge) in doses ten thousand times less than injectable interferon
- Binds to surface (mucosal) cells in the mouth/throat stimulating white blood cells and activates the immune system
- VELDONA® not aimed at a single disease or viral infection (as current antiviral and vaccine candidates), but used to activate overall immune system to prevent and treat a broad range of diseases and viruses such as Covid 19
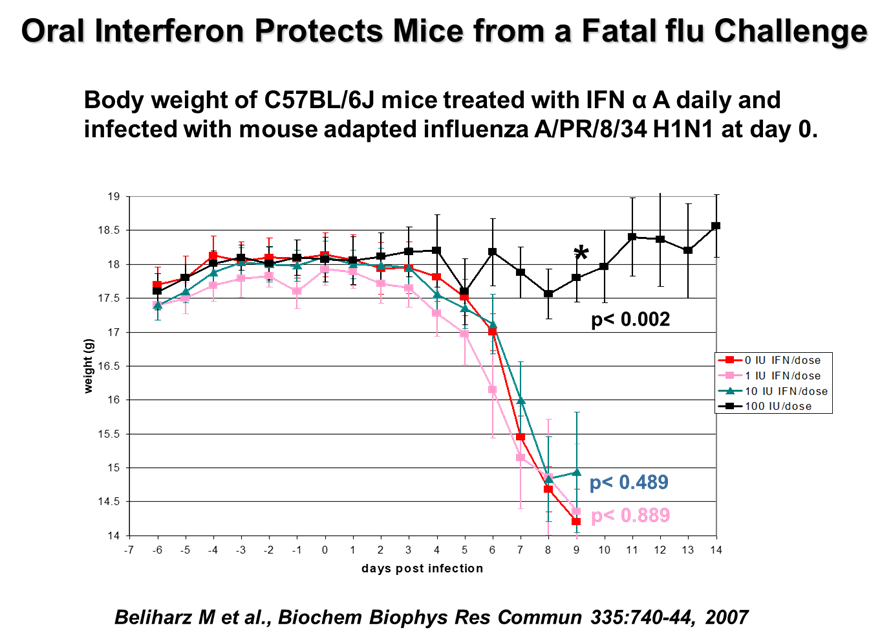
- The effectiveness of VELDONA® in research studies. VELDONA® was orally administered to C57BL/6J mice once a day. After 7 days, the mice were infected with human influenza virus (H1N1 virus) and the control group was given placebo to mice for seven consecutive days. For comparison, the oral low-dose VELDONA® group significantly reduced (P<0.05) virus replication in the lungs of mice and reduced death. (Refrence: Beliharz M et al., Biochem Biophys Res Commun 335:740-44, 2007)
Overview of GemOral®
- GemOral® is a product that encompasses three critical characteristics: Oral Formulation, Antiviral Potency, and Innate Immune Promotion which can promote the production of interferon and inhibit the production of inflammatory cytokine. GemOral® is tailored for COVID-19 treatment.
- With FDA approved API, Phase I & Ib clinical safety data, and GMP grade commercial level mass-manufacture capacity in the U.S., GemOral® is ready to launch a pivotal clinical trial for COVID-19 patients.
- By providing an oral, convenient, and early-stage treatment, GemOral® aims to offer a solution to slow down public viral spread, lower the cases of severe COVID-19, reduce healthcare burden, and eventually lead to a "same as flu" mortality rate.
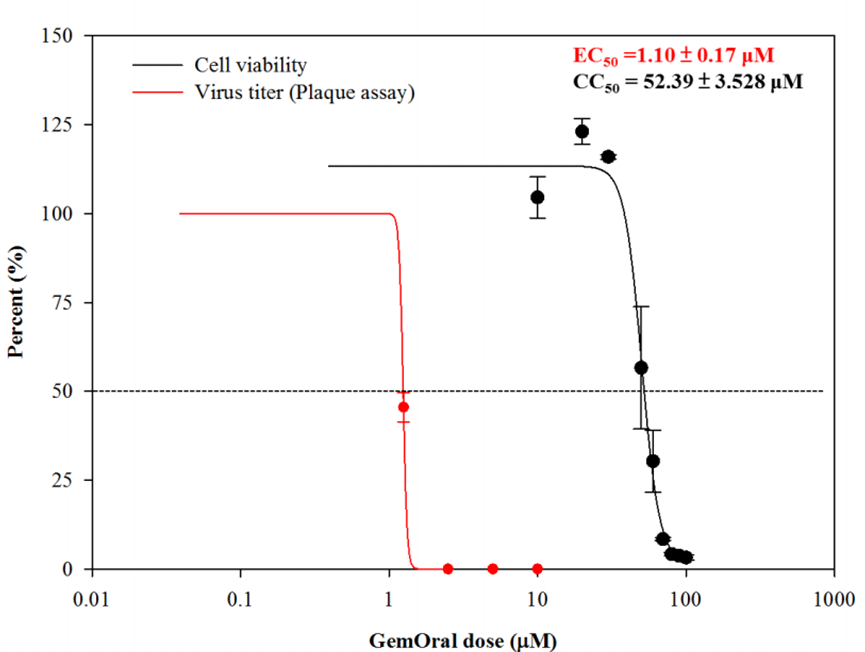
- GemOral® was commissioned by the National Taiwan University School of Medicine to conduct the Vero E6 cell test. The effective concentration (EC50) of the antiviral is about 1.1uM, and the concentration (CC50) that will damage the cells is about 52uM, which is about 48 times the effective concentration of the antiviral. It shows that GemOral® can kill a virus without harming the cells. The following chart shows the results of the test conducted by the National Taiwan University School of Medicine:
Brand Name | GemOral® |
Active Pharmaceutical Ingredient | Gemcitabine |
SKU | 40mg/20mg soft-gel capsule |
Manufacture Status | GMP grade mass-manufactured for clinical and commercial use in US |
Clinical Development |
|
Route of Administration | Oral Administration (P.O.) |
Regimen | Oncology(current): 1000mg/m2 intravenous infusion, 3 QW doses for a four-week cycle. Anti-Virus (proposed): 20mg oral administration, QD for 5 days. |
About Ainos, Inc.
Ainos, Inc., a Texas corporation (f/k/a Amarillo Biosciences, Inc.), is a diversified healthcare company engaged in the discovery and development of pharmaceutical and biotech products. The Company is currently focusing on point-of-care testing rapid test kit products that include diagnostics for COVID-19 (SARS-CoV2-Antigen Rapid Test), pneumonia, vaginitis and helicobacter pylori (H. pylori) bacterial infection. Ainos is transforming itself into a diversified MedTech leader through strategic relationships with innovative companies such as InnoPharmax. We are using our VELDONA® drug platform in our joint development arrangement with InnoPharmax. VELDONA® stands for very low-dose interferon alpha (IFN-α) that is orally administered and absorbed through the oral mucosa to initiate the innate immunity. VELDONA® is the company's registered trademark in Taiwan. For more information about the company please refer to: www.ainos.com
About InnoPharmax, Inc.
Innopharmax is a biopharmaceutical company focusing on the research and development platform of new oral drug formulations. Its main platform technology, OralPAS (Pro), can convert injectable drugs (such as insulin) into oral administration. The company develops and commercializes oral formulations with potential new drugs developed by the company or with its partners. InnoPharmax's vision is to become a leader in the development of new drugs and dosage-forms in Asia through formulation technology. Test results from cell experiments by Innopharmax show that GemOral® inhibits the infection caused by SARS-CoV-2 virus. The effective concentration (EC50) is about 1.1uM and the concentration (CC50) that causes damage to cells is 52.4uM. Gemcitabine, the main component of D07001, promotes autoimmunity at low concentrations which increases the secretion of interferon and reduces the secretion of inflammatory cytokine. Gemcitabine, which is same as Merck's oral drug -MK4482, is an analog of cytidine that inhibits the replication of virus in cells and has high activity on cancer cells. Innopharmax has completed the Phase I clinical study and plans to move forward to Phase II/III. InnoPharmax's drug development and product lines include anti-infective drugs, cancer drugs, immune drugs, antidiabetic drug and rare disease drugs. GemOral® is the company's registered trademark in Taiwan. For more information about the company please refer to: www.innopharmax.com
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This press release contains 'forward-looking statements' within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as "anticipate," "believe," "estimate," "expect," "intend," "plan," "predict," "project," 'target," "future," "seek," "likely," "strategy," "may," "should," "will," and similar references to future periods. Forward-looking statements are based only on our current beliefs, expectations, and assumptions. Forward-looking statements are subject to inherent uncertainties, risks, and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results may differ materially from those indicated in the forward-looking statements.
Important factors that could cause our actual results to differ materially from those indicated in the forward-looking statements include, among others, the following: the cost of production and sales potential of the planned drug treatments announced in this press release; the impact of final approvals from the U.S. Food and Drug Administration (the "FDA") or other regulatory bodies for the planned drug treatments including the availability of emergency use authorization; the Company's limited cash and history of losses; the Company's ability to achieve profitability; intense competition and rapidly advancing technology in the Company's industry that may outpace its technology; customer demand for the products and services the Company develops; the impact of competitive or alternative products, technologies and pricing; the Company's ability to manufacture any products it develops; general economic conditions and events and the impact they may have on the Company and its potential customers, including but not limited to the impact of Covid-19; the Company's ability to obtain adequate financing in the future; the impact of promulgation and implementation of regulations by the World Health Organization, the FDA and by other governmental authorities with functions similar to those of the FDA on the Company's operations and technologies; lawsuits and other claims by third parties or investigations by various regulatory agencies governing the Company's operations; the Company's ability to secure regulatory approvals for its products; and our success in managing the risks involved in the foregoing items. Readers should also review the risks and uncertainties listed in our most recent Annual Report on Form 10-K and other reports we file with the U.S. Securities and Exchange Commission.
Any forward-looking statement made by us in this press release speaks only as of the date on which such statement is made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.
Contact:
Ainos, Inc.
Lawrence Lin
Tel: (858) 869-2986
Email: lawrence@ainos.com
SOURCE: Ainos, Inc.
View source version on accesswire.com:
https://www.accesswire.com/676383/Ainos-Announces-Strategic-Relationship-with-InnoPharmax-to-Jointly-Develop-Combined-Oral-Therapy-for-Coronavirus-Infection





