IRVINE, Calif. - September 28, 2020 - (Newswire.com)
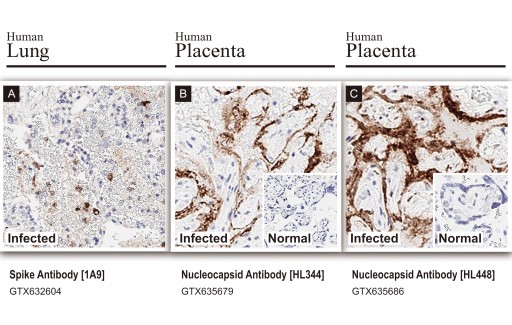
GeneTex, Inc., a leading research antibody manufacturer, has developed the most comprehensive and well-characterized catalog of SARS-CoV-2 reagents for researchers. In an effort to further optimize these antibodies, GeneTex has partnered with HistoWiz, Inc., which specializes in histopathological services. The first phase of this collaboration has established several highly validated antibodies for immunohistochemistry (IHC) on human SARS-CoV-2-infected tissues (see GTX632604 COVID+ Lung, GTX635679 COVID+ Placenta, GTX635686 COVID+ Placenta).
The first of the three validated antibodies is the mouse monoclonal [1A9] clone (GTX632604) that recognizes SARS-CoV and SARS-CoV-2 spike proteins. This antibody has already been published in multiple COVID-19 studies. The other two reagents are recombinant rabbit monoclonal antibodies targeting SARS-CoV-2 nucleocapsid protein (clones [HL344] (GTX635679) and [HL448] (GTX635686)), with GTX635686 demonstrating unprecedented sensitivity for IHC. Both of the nucleocapsid antibodies were engineered in GeneTex’s new, state-of-the-art recombinant antibody facility and are validated for several other applications, as is [1A9].
HistoWiz’s rigorous validation of these three antibodies for IHC has come at a crucial time as researchers strive to learn more about SARS-CoV-2 and COVID-19’s multi-organ involvement. Dr. Ke Cheng, CEO of HistoWiz, remarks, “For many years, HistoWiz has been accelerating histopathology for researchers in academia, industry and government institutions including the CDC. We are excited to have developed the first automated IHC assay with GeneTex to detect active SARS-CoV-2 viral infection in a variety of tissues. We are dedicated to taking part in the fight to end this pandemic.” IHC identifies active viral replication by detecting viral proteins in infected tissues, unlike RT-PCR which only amplifies fragments of the viral RNA in the sample specimen.
Dr. Alex Ball, Senior Scientist at GeneTex, comments, “Remarkable discoveries about SARS-CoV-2 biology and COVID-19 have come so quickly due to the tireless efforts of researchers, to the point that therapies and vaccines will likely soon be available. Nevertheless, scientists will want to delve deeper into SARS-CoV-2-related organ pathology, so there is urgent need for reliable IHC-validated antibody reagents to facilitate this research.”
As GeneTex continues to expand its SARS-CoV-2 antibody portfolio, its collaboration with HistoWiz has established the groundwork for the development and IHC optimization of additional antibodies, which will hopefully be available to the research community within the coming months.
GeneTex products are for research use only. Not for diagnostic or therapeutic procedures.
Media Contact:
Allen Lee
Phone: 949.553.1900
Email: allensl@genetex.com
Related Links
GeneTex
HistoWiz
Press Release Service by Newswire.com
Original Source: GeneTex SARS-CoV-2 Nucleocapsid and Spike Antibodies Independently Validated by HistoWiz for Immunohistochemistry
